SAM vaccine for H7N9
Greetings,
Influenza, continues to be a serious issue. With emergence of a new pathogenic strain- H7N9 in china (Read previous post here and here), influenza research focussed on making a vaccine. Virtually nothing much is known about this strain except for its mutation that has conferred a gain of function. On 23 April, China's state news agency reported a 36-year-old man in serious condition in the city of Zaozhuang. The rest is history. The H7N9, is a deep reflection of how less we know of the influenza virus.
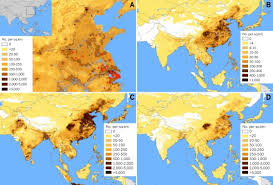 |
Fig 1: H7N9 cases to 16 April 2013 (red circles),
and population densities of humans. Nature news
|
A little bit of digression, related to H7N9 studies. If you remember the story of influenza ferret experiments (Link) that dramatically unfolded over time (Link), it has been clear that gain of function (GOF) experiments is under high scrutiny by the public, for most of them don't understand what actually is the nature of experiment. The issue scaled up to a global news with intervention of Federal agencies. Though the paper was finally published and made available in open access format, the trouble of publishing had probably got scientists thinking along. So when a group of scientists planned to do some similar GOF experiment, the public and science is on a debate with opinions broadcasted or publicly posted. This was deliberately done to allow open discussion, so as to avoid future interference problems (Probably!!).
Influenza vaccine is a subject of huge research. New Influenza strains of interest arise with un-predicted antigenic combinations, which delays the vaccine production. Rapid production technologies such as the RNA vaccine approach (CureVac’s RNActive ® technology; Link), attempts for universal vaccine, cell-free gene assembly technique, DNA vaccines etc have gained a huge interest. So when the Nature published SAM vaccine for H7N9, I had to blog about it.
This research is very much based on a technology referred as self-amplifying mRNA (SAM™) platform for rapid synthesis of vaccines. Technique is based on self amplifying RNA delivery, using lipid nanoparticles (LNP). Several papers have been published were a non amplifying RNA (In this case siRNA) delivered to cells through LNP was used to treat allergy, neoplasms etc. The basic structure of a SAM is shown in Fig 2. The gene of interest (Whatever is the protein wish to be coded for) can be inserted, bingo. In a paper by Geall etal, a proof of concept experiment was shown to be highly successful. The experiment used self-amplifying RNA vaccines called stable nucleic acid lipid particles, delivered using DLinDMA (ionizable cationic lipid 1,2-dilinoleyloxy- 3-dimethylaminopropane).
HA gene was synthesized from the A/Shanghai/2/2013, subsequently cloned into SAM vaccine genetic control elements and a T7 polymerase promoter. The copy was transcribed and transfected to BHK cell lines, checked for integrity and expression patterns. Finally, it was packaged into the LNP delivery system (Now referred completely as SAM/LNP), and tested against mice. The total time taken from identification of sequence to making of SAM, just 8 days (Refer Fig 3). Thats the fastest I have ever heard. By the way they tested its activity against mice and showed a good quality response, with 2 doses. Quote from article "With further streamlining, it should be possible to produce similar vaccine candidates within 5 days of the gene sequence of the virus being available".
As the article points out, this methodology has several advantages. The most important is use of cell free system to synthesize the HA gene (nucleic acid amplification technologies such as PCR. or isothermal techniques), and use of SAM technology to create vaccine with speed and accuracy. The only disadvantage is the RNA half life, which requires some care.
Take home message, if we are not prepared to battle when the virus appears, we can at the fastest possible time, with SAM technology.
References:
Influenza vaccine is a subject of huge research. New Influenza strains of interest arise with un-predicted antigenic combinations, which delays the vaccine production. Rapid production technologies such as the RNA vaccine approach (CureVac’s RNActive ® technology; Link), attempts for universal vaccine, cell-free gene assembly technique, DNA vaccines etc have gained a huge interest. So when the Nature published SAM vaccine for H7N9, I had to blog about it.
 |
| Fig 2: Self amplifying mRNA derived from Alphavirus |
HA gene was synthesized from the A/Shanghai/2/2013, subsequently cloned into SAM vaccine genetic control elements and a T7 polymerase promoter. The copy was transcribed and transfected to BHK cell lines, checked for integrity and expression patterns. Finally, it was packaged into the LNP delivery system (Now referred completely as SAM/LNP), and tested against mice. The total time taken from identification of sequence to making of SAM, just 8 days (Refer Fig 3). Thats the fastest I have ever heard. By the way they tested its activity against mice and showed a good quality response, with 2 doses. Quote from article "With further streamlining, it should be possible to produce similar vaccine candidates within 5 days of the gene sequence of the virus being available".
 |
| Fig 3: Timeline of SAM H7N9 vaccine production. Source |
Take home message, if we are not prepared to battle when the virus appears, we can at the fastest possible time, with SAM technology.
References:
Malakoff D (2013). Avian influenza. Critics skeptical as flu scientists argue for controversial H7N9 studies. Science (New York, N.Y.), 341 (6146) PMID: 23926188
Geall AJ etal (2012). Nonviral delivery of self-amplifying RNA vaccines. PNAS, 109 (36), 14604-9 PMID: 22908294
Armin Hekele etal (2013). Rapidly produced SAMH vaccine against H7N9 influenza is immunogenic in mice. Emerging Microbes and Infections 2, e52. doi:10.1038/emi.2013.54





Comments
Post a Comment