NextGen vaccination: Needle free devices, Interleukin adjuvants
Greetings
I have visited the topic of vaccination in this blog many different times. Vaccines are the safest and cost effective solution for many different infectious diseases. Approximations state, vaccination has saved a minimum of 35-40% of world population from developing fatal conditions. Problems still does exist in creating a safe and effective vaccine for infectious conditions (such as Tuberculosis, Staphylococcus aureus, HIV, Plasmodium, Influenza etc ), though efforts are made to improve. Vaccination for HPV (Gardasil) is a classic example, of a successful vaccine campaigning against cervical cancer.
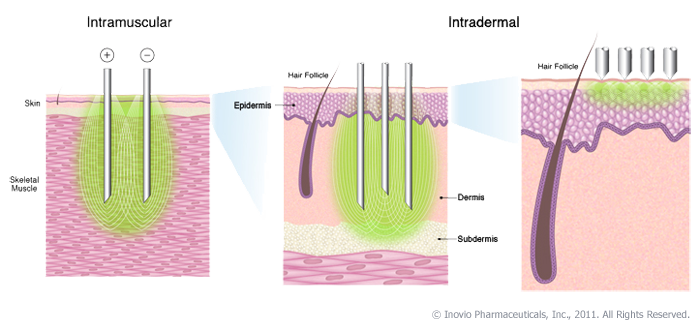 |
| Fig 1: Inovio Vaccine delivery. Source |
With the same concept, IL 33 has been used to boost the HPV vaccine powers. As Dr. Kim puts it, "We are developing multiple DNA plasmid based cytokine and chemokine genes as immune activators and I am proud to say that Inovio has more of these immune activators in its pipeline than anyone else in the world. We have initiated human studies using other DNA-based cytokines and look forward to moving IL-33 into clinical trials in combination with our DNA vaccines". Source
 |
| Photo 2: Vaccine-Delivery Patch. Source |
The second important factor is the use of adjuvants. There is still a debate as of how the adjuvants work. In simplest terms, adjuvants are substances that increases the immunological response to an antigen. You can find an in depth discussion of the adjuvant mechanism of action here. I want to note, the use of interleukins themselves as a booster of immunity is a very new concept.
Taking example from above, Il 12 has been shown to induce improve specific response against HIV. This maybe explained by, IL 12 is a central regulator of the cell mediated immunity. Similarly, the use of IL 28 maybe explanied by, its role in immune defense against viruses, important factors such as ISGF3G (Interferon Stimulated Gene Factor 3), Mx proteins etc. The use of IL 33, which is involved in regulation of helper T cells and promotes type 2 cytokine functions can enhance effects of antiviral and anti neoplastic cell activity which may explain the success.
Take home message is immunity is a very delicate issue. A lack of immune response in one type of vaccine is not a proof of non antigenicity. Probably we have to test the right cocktail, and interleukins seem to be good candidates.
Kalams SA etal (2013). Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. The Journal of infectious diseases, 208 (5), 818-29 PMID: 23840043
Villarreal D, Wise MC, Walters JN, Reuschel E, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, & Weiner DB (2014). Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer research PMID: 24448242
Kichaev G, Mendoza JM, Amante D, Smith TR, McCoy JR, Sardesai NY, & Broderick KE (2013). Electroporation mediated DNA vaccination directly to a mucosal surface results in improved immune responses. Human vaccines & immunotherapeutics, 9 (10), 2041-8 PMID: 23954979
Gherardi MM, Ramírez JC, & Esteban M (2001). Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens. Histology and histopathology, 16 (2), 655-67 PMID: 11332721





Comments
Post a Comment